Note for: Clinically Relevant Concentrations of Anticancer Drugs: A Guide for Nonclinical Studies
Note for: Clinically Relevant Concentrations of Anticancer Drugs: A Guide for Nonclinical Studies
Doi: 10.1158/1078-0432.CCR-16-3083
Aim of this paper;
Giving a guideline for setting up the in vitro experiment on cell line, esp. concentrations by which it makes some sense when translated back to clinical level.
Extraction;
For doing experiment with FDA-approved drug – it is better to search for pharmacokinetics as well as toxicity of particular drug. Thus, we can design the experiment which will be more clinically relevant.
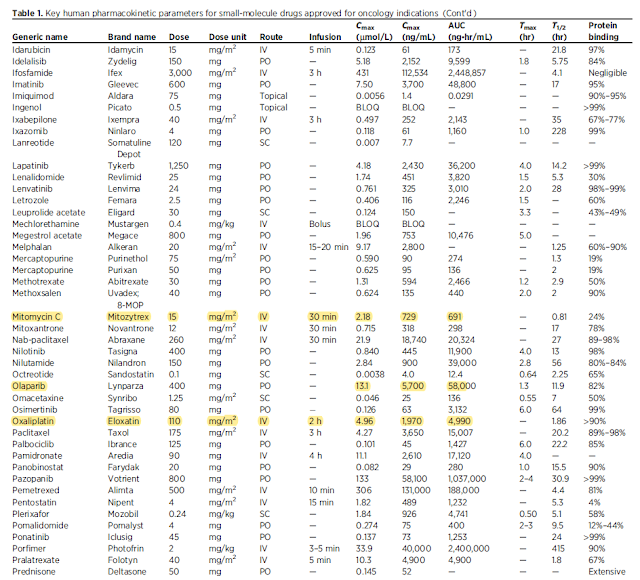
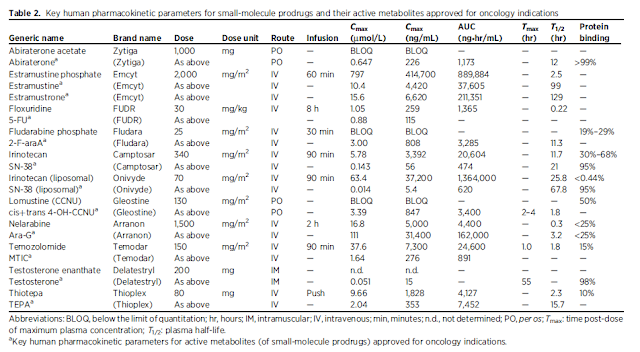
Guiding dose and concentration selection – providing comprehensive compilation of human plasma exposures for drug-approved by the FDA for used in oncology.
Sources of FDA-approved drug
NCI – cross-checked with MediLexicon and Centerwatch
Pharmacokinetic search through databases – original literature or conference abstract
Using Cmax (maximum plasma concentration) and the integrated area under the plasma concentration–time curve (AUC) associated with the highest recommended dose of the drugs
Results
145 unique small-molecule drugs approved to treat cancer
10 – prodrugs
Active drugs (table1)
IV route – duration of injection is included
Cmax – normally reported as ng/mL but convert to umol/L
Fraction bound to plasma protein – important parameter to translate from in vivo to in vitro setting with varied protein composition
Cmax and AUC presented here – average values and interindividual variability can be large due to genetic polymorphism in clearance and other factors
Attempt to translate doses or plasma exposures from nonclinical models to human – most utilize
allometric scaling/empirical measurement
based on body surface area
in vivo pharmacokinetics
pharmacokinetics/pharmacodynamics and physiologically based pharmacokinetic models
Plasma Cmax – highly dependent on
route of administration,
formulation and
physical properties
Cmax
May consider as an upper limit for drug concentration during in vitro studies
May consider as the highest plasma exposure for in vivo studies
This will help the off-target effects during study in vivo
10 or 100 times greater than IC50 or Ki for the molecular target – increase the possibility of introducing off-target activity – it will be unrelated to clinical benefit
Cmax – (free + protein bound); free form is the form which interacts with molecular target – it is important to consider protein binding percentage before setting up the experiment
Each drug has different serum protein binding capability – thus the mode of action will be affected
Species different in plasma protein binding – effect the binding capability to each drug
For example, vismodegib primarily bound to alpha-1-acid glycoprotein (AAG) with much lower affinity for albumin
Kd between rat and human albumin-AAG yields 100-fold differences
AUC
Integration of plasma drug exposure over time – things need to be considered
Bioavailability
Different absorption rates
Elimination rates
Particularly important when comparing exposures between species or routes of administration
Things to be considered
Cytochrome-P450 isoform – produce different metabolites might be more potent than the parental
Route to introduce drugs in animal – ip in mouse, mostly, being absorbed through the visceral peritoneum
Route to introduce drug – must consider first-pass hepatic clearance







Comments
Post a Comment